Aisiri Amin
|
March 3, 2026
|
12
min read
Bibijan Halemani dared to dream—of millets, and a better world for women
Halemani’s award-winning self-help group in Karnataka runs a seed bank and instills new confidence in millet cultivation
Read More

Halemani’s award-winning self-help group in Karnataka runs a seed bank and instills new confidence in millet cultivation
Editor's note: Every farmer who tills the land is an inextricable part of the Indian agriculture story. Some challenge convention, others uplift their less privileged peers, others still courageously pave the way for a more organic, sustainable future. All of them feed the country. In this series, the Good Food Movement highlights the lives and careers of pioneers in Indian agriculture—cultivators, seed preservers, collective organisers and entrepreneurs.
On a Sunday morning in September 2025, in Teertha—a small village about 30 km from Karnataka’s Hubli region—Bibijan Halemani makes her way home. A man sitting near a corner store asks her, “Will you buy mekke beeja (maize seeds) this time?”
Before she can respond, another man has a ready answer: “They have won a prize. She can.” Halemani smiles and continues walking.
“People are talking about the prize now, but until recently, they had little faith in our self-help group (SHG),” she says. The prize she speaks of is the Equator Initiative Award, won by the Bibi Fatima Self-Help Group for community-led biodiversity conservation, meaningful work in food security, and creating jobs for marginalised women.
“Recognitions like these make it difficult to dismiss our [women’s] work,” she says. However, it isn’t the first one. In the last seven years, the SHG has received several recognitions; of these, the one that first changed the way people looked at them was Deccan Herald’s Changemakers Award in 2023.
As the 40-year-old reaches the SHG’s community seed bank, started in 2018, which is next to her house, two sparrows are chirping around in the verandah. “You’ll always find sparrows here. They love seeds.” As she opens the door, the birds rush in, happily flying in circles near the roof.

With earthy-red hand-paintings across the white walls and millet husks all around, the seed bank stands out from the line of houses surrounding it. Near the entrance, three rows of baskets full of millets—from the popular kodo and foxtail, to the lesser known browntop–greet us. Next to them, some millet foods such as sevai (vermicelli noodles) and beaten millet rice flakes are displayed on a wooden table where Halemani keeps tea and snacks for visitors. As we enter, the wall opposite the door is lined with various colourful seeds and millets in small glass bottles, along with the awards that the SHG has won.
Within ten minutes of meeting Halemani, three phone calls have already interrupted the conversation. Her busyness is also reflected in her way of talking: fast and to the point. But life wasn’t always like this for her, she recalls.
“About 20 years ago, if someone had told me this is what I will be doing, it would be a little surprising,” she says. For Halemani, ambition came with constant reminders of her gender identity. “The women in my family didn’t really have the option to study a great deal, or work—or even step out of our homes,” she says. Today, her work routinely takes her far away from Teertha, her hometown and address after marriage, to Delhi and Maharashtra.
A ghost ship, carrying dreams of a different life, often makes its presence felt in Halemani’s world. In her late teens, she developed a deep interest in politics and social work. “I just wanted to do something for society,” she says softly. When she completed her degree in Politics, Hindi, and English, her sole focus was on doing a Bachelor’s in Education to fulfil a dream she had stubbornly kept alive. “I always wanted to become a teacher,” she says.
Although acutely aware of the restrictions that had always been imposed on her, she hoped that this could be possible. However, her parents saw no point in further education or work, and got her married off as soon as she turned 20.
For Halemani, ambition came with constant reminders of her gender identity.
In 2004, soon after her wedding, Halemani tried to chase after this dream and applied to become an Anganwadi teacher in her village–despite a lack of any support. “But people in my village were opposed to my application, likely driven by internal politics and a bias against educated women. They made sure the position went to a woman from another village. After that, I gave up on the idea,” she says.
After marriage, her movement and access to public spaces shrunk further. Her days mostly revolved around cooking for many, tending to the cattle, and making manure out of cattle dung. During this time, she observed farming more than ever before—from what people chose to cultivate, to the problems farmers faced. One such observation was the disappearing presence of millets from plates and fields. “After 2007, not many were growing millets. Farmers were switching to maize, cotton, and rice,” she says.
In 2017, when the NGO Sahaja Samrudha came to her village to talk about farming challenges, men and women were both encouraged to come to the meeting; this marked her introduction to sustainable farming. “They noticed that women were more active and vocal during the meeting, and approached us with the idea of a self-help group focused on promoting millet farming,” Halemani explains. Seated next to baskets of millet grains, she adds, “It changed our lives.”
Also read: In Leh’s harsh terrain, farmer Urgain Phuntsog is a true ‘mitti ka aadmi
Halemani and 14 other women from Teertha came together to form the Bibi Fatima Self-Help Group in 2018. This was uncharted territory for them all. “People don’t have much land here. Some women would travel to faraway places to earn money. A few from our SHG used to catch a bus at 6 in the morning on chilly, winter days to wash utensils at a resort for just Rs. 300 and return only in the evening. They would fall sick, but still work,” Halemani says.
For years, Teertha’s women tried to find different sources of income. “We have to work constantly,” she says. In 2004, a few women, including Halemani, started an SHG to put aside a portion of their earnings and save it for a rainy day, but it had limited success, and they had to close it three years later.

When the Bibi Fatima SHG was formed, for the very first time, the women had guidance and mentorship. They didn’t really know much about biodiversity conservation or food security, but through training programmes organised by Sahaja Samrudha, they started connecting their lived experience and observations with scientific knowledge.
When the Bibi Fatima SHG was formed, for the very first time, the women had guidance and mentorship.
The initial years also brought scepticism and stigma, as members of the SHG were often scoffed at. “If I carried a shoulder bag, some people would mock me, saying that I am ‘showing off’ my work. Now the same people are congratulating us,” Halemani says.
In particular, she remembers how disrespectfully the women were treated at a local bank when Halemani and her peers wanted to apply for a bank account for their undertaking. The manager, peeved at Halemani for taking a phone call, mocked her—an anecdote that resonates with many women, especially those who aren’t educated, and who feel hesitant to enter banks.
Also read: How Rahibai Popere built a seed bank with a mother’s grit and love
“Siridanya (millets) are not new to us. We have been exposed to them even as children, but I didn’t know of their benefits to health or the environment. Now, I am able to talk to others about millets and make them aware, too,” Halemani says with a smile—the first in this conversation.
Most farmers in and around Teertha had long left behind millet cultivation by the time Sahaja Samrudha put forth its idea. “Farmers were not finding it profitable. The process of cleaning millets after harvests was an issue for many,” she says.

The situation in Teertha was a microcosm of what was underway across Karnataka. By 2017, the abandonment of millets became a growing issue spurring state-wide concern. During the All-India Co-ordinated Project on Small Millets in April 2017, H. Shivanna, the Vice-Chancellor of the University of Agricultural Sciences, Bengaluru, said that over the preceding decade, Karnataka had lost nearly 2.5 lakh hectares of millet-growing fields to acacia and neem. He added that 40% of the area that was under millet cultivation had been replaced by horticultural crops, which meant that the state had just about 9.5 lakh hectares dedicated to growing millets.
Where once millets had disappeared from the fields in and surrounding Teertha, in present times, farmers are cultivating them across about 2,000 acres of land.
To address this gradual decrease, and after consecutive droughts between 2013 and 2017, the Karnataka government focused on reviving production. They also organised awareness programs and promotion campaigns in and around Bengaluru. In 2017, the National Organic and Millets Fair was held to bring together stakeholders in millet production and to connect farmers to markets. The government also pushed for 2018 to be declared as the ‘National Year of Millets.’
However, when it came to rural Karnataka, it was SHGs such as Halemani’s that heralded millet production. The Bibi Fatima SHG took flight in 2018. In the first two years, the women went to different taluks around Teertha to visit farms and meet farmers, explaining the cost benefits that come with growing millets and informing them of training and welfare schemes on offer. A particular advantage of growing millets is the crops’ ability to adapt well to different environmental conditions, especially changing monsoon patterns—a change that farmers are taking notice of. “In recent times, it rains heavily during the non-rainy season, and we barely get rain when we are supposed to,” Halemani says.

Yet, farmers remained sceptical. They wouldn’t come to the meetings or show much interest. The SHG then started giving farmers Navdanya kits–free packs provided by Sahaja Samrudha that consisted of nine kinds of millets that can be grown in a one-acre field. Gradually, more farmers showed a willingness to experiment.
As awareness grew and millet cultivation became more lucrative, more farmers joined the group. “We started with 20 to 25 farmers, but now we have a network of 5,000,” she says. This growth has largely been the result of the sharing of personal testimonies and success stories. Where once millets had disappeared from the fields in and surrounding Teertha, in present times, farmers are cultivating them across about 2,000 acres of land.
To address the gap between harvests and processing—the cumbersome cleaning of the grains—the SHG set up a millet processing unit in Teertha in 2022, with the help of Sahaja Samrudha, the Indian Institute of Millets Research (IIMR), Hyderabad, and CROPS4HD–an international project set up to transform food systems. The unit is run completely by the women. The SELCO foundation also provided them with solar power for their unit, which helped them manage their electricity expenses. The Millet Foundation, Bengaluru, trained the women in operating the unit, where they now earn Rs. 500 for each day of work.
Halemani gets up to reach for the millet baskets, holding a few grains of the browntop millet—small and polished, shining soft-golden in the light. “See, it has to be cleaned and properly processed for it to look like this,” she says. Processing is crucial in removing the inedible parts and increasing the bioavailability of nutrients. “Once we collect the seeds from the farmers, germination determines their quality. Those that germinate at least 80% are stored as seeds in the bank, and the rest are processed to make millet rice,” she explains.

Farmers from about 15 villages get their grains processed at the SHG’s unit, where small quantities are undertaken for farmers’ consumption, as well as purchases in bulk for the market. In 2024, about 50 metric tons of millets were processed. The most palpable impact of this ease of processing is an increase in the household consumption of millets, Halemani says. “When we started, no one in our village was eating millets, but now at least 25% eat millet rice daily,” she adds.
To expand their reach, 53 SHGs came together in 2023 to form Devdanya Farmer Producer Company, a farmers' producer organisation (FPO) which is co-led by Halemani. About 500 individual farmers from the Kundagol and Shiggaon taluks are part of this initiative. In its first year, the FPO registered an annual turnover of Rs 58 lakh. After excluding all expenses and paying taxes, it made a profit of over Rs 1.4 lakh. “In 2024, our annual turnover increased to Rs. 1.5 crore,” Halemani shares.
While Devdanya was set up to promote millet products such as health drinks, sevai, and rice, it was also intended to remove the middlemen between farmers and markets. “The rate for millets is set by middlemen, and if these rates are too low, farmers are disincentivised from growing these crops. Through Devdanya, we buy the millets, help farmers process them, and ensure they have enough for household consumption.”
Also read: Babulal Dahiya makes every grain of rice count
Building a community seed bank has been a core project of the SHG since its very inception. When they were looking for a place to set it up, Halemani offered the space next to her house, which belongs to her family. “After about a year of creating awareness about sustainable farming and distributing Navdanya, we began to receive seeds in return. If we gave 10 kg of seeds, the farmer had to give us 20 kg back. That’s how the community seed bank started,” she says.
Initially, it received only millets, but as the harvests expanded, so did the seed bank. Today, it is home to 350 varieties of millets, oil seeds and pulses. These include 74 varieties of ragi, 10 of foxtail millet, 25 of little millet, two of proso and browntop, one of barnyard and pearl each, as well as 25 varieties of pulses and 80 of vegetables. Currently, the SHG recognises 30 farmers as seed producers.

The core idea behind the seed bank, Halemani shares, has been to increase farmers’ access to indigenous varieties and provide them free of cost. They can usually find what they require here, depending on the season. With the climate crisis looming, the group has prioritised seeds which can withstand extreme environmental changes––mainly millets.
However, it hasn’t been an easy path for the SHG. Building a relationship with farmers was an exercise in time and patience. Sometimes, the SHG wouldn’t receive the seeds they had lent, and farmers would return millet seeds that wouldn’t germinate. “There were also times when we didn’t have the seeds that farmers needed, so we used our savings to buy them. There have been challenges, but isn’t that how life is?” says Halemani.
Though this may seem marginal, it is a triumph for a community-oriented seed bank in India, where farmers who run such institutions often rely on their own funds or meagre donations.
The community seed bank has been widely recognised for its focus on indigenous seeds, food security and the promotion of sustainable farming. “In 2024 we made transactions of up to Rs.14 lakhs, but in 2025, we made about Rs.10–11 lakhs because we faced some technical issues with the machines that processed millets ,” Halemani explains. They only make an estimated 10% of profit on their earnings, as they have to bear the cost of labour, rent and other expenses. Though this may seem marginal, it is a triumph for a community-oriented seed bank in India, where farmers who run such institutions often rely on their own funds or meagre donations.
At around 1 PM, Halemani generously extends an invitation to join her family for lunch and refuses to hear anything other than yes. As we sit down, she first brings a plate of millet sevai, with milk and sugar on the side. It’s a simple, wholesome meal, popular in Uttara Kannada. The sugar is sprinkled on the sevai, and milk is poured on top. Then come rice, sambar and sandige (sun-dried fritters). “We eat jolada rotti (rotis made of sorghum), but I didn’t think they’d be familiar to you,” she says. As we dig in, another member of the SHG, who is also Halemani’s relative, joins us. “She was the first to be a part of the group,” Halemani explains, nodding towards Shehanajabi Halemani, 42.
Shehanajabi has made a living from tailoring for a long time, but considers working at the SHG as her first and primary job. “Since the SHG was established, there is some work or other which keeps us busy, so the opportunities to earn have increased.” Shehanajabi adds, “We also help farmers and the environment, so the work feels fulfilling.”
For many women in and around Teertha, dreams have felt like a luxury that they don’t have access to.
When asked if she had a dream for her life while growing up, Shehanajabi shrugs and smiles as she looks into the distance. For many women in and around Teertha, dreams have felt like a luxury that they don’t have access to.
As we make our way back to the seed bank after lunch, we are in the company of another SHG member: Prema Prabhakar Bollina, 28, who is with her two-year-old daughter. Bollina got married when she was a teenager and is now a mother to two children. “I wanted to study. I think I could have become a teacher,” she says, with a big smile that stubbornly stays on throughout the conversation. Before she joined the SHG in 2019 and began working at the seed bank, Bollina was a domestic worker. As one of the few women in the area who is educated, she largely works at the bank, undertaking administrative tasks like registering transactions. “Earning recognition has changed the way people look at us. It has also made us independent,” she says.
Earlier that day, Halemani proudly showed photographs of her children: a 20-year-old son, who is studying law, and an 18-year-old daughter, who is pursuing a paramedical degree. “Ever since they were children, I told them education is vital to make something of their life,” she says. Even as their own dreams may have been made inaccessible, Halemani, Bollina and other women in the SHG are fiercely protecting the aspirations of their children.
Edited by Neerja Deodhar and Anushka Mukherjee
Art by Jishnu Bandyopadhyay
{{quiz}}

The hands that bring us India’s favourite beverage are under-nourished as they battle anaemia and rely on foraged produce for their health
Whether they’re sweating it out in the harsh summer–with the humidity hovering above 80%–or standing tall in a heavy downpour, a tea garden worker in Assam has a set target. They are expected to pluck at least 25 kg of tea leaves each day, for a daily wage of around Rs. 200. This reality persists across 800 or so plantations throughout the state. The same tea leaves easily fetch Rs. 50,000 in private auctions after they’ve been processed. The chasm between pay and price only gets wider in the case of specialty tea: In 2022, a single tea estate set a record when it sold the rare, valued Manohari gold tea from the Dibrugarh district at the rate of Rs 1.15 lakh per kg.
What sustains these workers, who pluck some of the costliest tea leaves in the world, to work eight-hour shifts in extreme weather with only an hour’s break for lunch? The answer, tragically, is very little. Our research threw up some uncomfortable truths: Out of 14 lunch-and-dinner meals in a week, most families consumed only two meals with adequate nutrients. Across Assam’s estates, more women are engaged in tea plucking than men. Endless studies highlight the alarming rates of anaemia in these women; prominently, a sample study from the UNICEF finds that 95% of women working in tea gardens are anaemic. The data is grim. Conditions like anaemia can be the result of various factors, but a reigning influence is nutrition.
There are no easy answers to be found to questions about what their daily diets consist of. But the history of Assam’s tea estates is a good place to start.
Assam’s tryst with tea started with the advent of the East India Company’s stronghold in the region in the early 19th century. The Treaty of Yandaboo in 1826 initiated British rule in northeastern India, setting up solid territorial boundaries for the first time. All of this was happening against the backdrop of a race between the British and the Dutch to control tea production in parts of eastern India.
For a long time, almost all the world’s tea came from China–while the British and Dutch raced frantically to establish some hold over the production and export of this highly demanded commodity. All of this changed when Robert Bruce, a British merchant, discovered indigenous tea plants growing in the Brahmaputra Valley. The moist evergreen rainforests of Assam and Bengal had always been home to wild tea trees, which were harvested by native communities on the backs of elephants. Much like the ancient Chinese, these communities revered tea leaves for their healing properties. When the British confirmed that these leaves could be commercially cultivated across the perfect slopes of these regions, they set up an entire industry.
In its early years, Assam’s tea industry had plenty of demand globally, but not enough local, skilled labour to work in the plantations. As a result, several tea estates suffered losses and were forced to shut down. Working on a tea estate was an extremely labour-intensive job, and Chinese workers who were brought in from across the border demanded a fair wage for their skill.
Over a century, as these amalgamated tribes toiled hard in the tea estates, they could hold on to very little of what they brought from their ancestral lands, in terms of their diverse culture, food or even languages.
Instead of giving in to their demands, British tea estate owners employed a network of agents (called arkuttis) who travelled to neighbouring states, found men and women who were desperate for a means to survive, and promised Assam to them as a land of opportunity. However, the compensation and treatment of these workers, once they travelled to Assam, was no less than that meted out to bonded labourers.
Also read: For Assam's Mising community, this fish paste represents tradition and food security
The British indentured mostly Adivasi people from Central India, who belonged to communities like the Oraon, Munda, Ghasi, Santhal, Tanti, Bhumij, Karmakar, Lohar and Sahu. By 1901, over 7 lakh skilled workers had migrated to Assam to work in tea gardens, with over 5 lakh of them from the Bengal Presidency alone.
The amalgamation of these communities in the tea-growing districts gave birth to what is colloquially called as Baganiya culture (roughly, the garden culture). The Baganiya culture soon gave birth to the Baganiya creole, borrowing words from Central Indian tribal languages. The descendents of these migrated labourers in Assam are now often called ‘tea tribes.’
Over a century, as these amalgamated tribes toiled hard in the tea estates, they could hold on to very little of what they brought from their ancestral lands, in terms of their diverse culture, food or even languages. Assam became their new home.
{{marquee}}
We met Renu Oraon (name changed to protect identity), a fulltime worker in a large estate in eastern Assam’s Golaghat district. A part of the organised workforce of the tea industry–protected by a labour union–Oraon earns around Rs. 1,200 for six days of work that start as early as 4 am on some days. We followed her through an exhausting 24 hours: Typically, her shift begins at 8 am and is broken into two by a 45-minute lunch break. She can only attend to chores at home after 5 pm. Her routine is arduous and takes a massive physical toll; Oraon can only sleep after 9 pm.

Workers reside in the ‘labour lines’: rows of two-room houses—barely 25 sq. feet—provided to them by the estates. Oraon says that 22 houses in the labour line, including her own, do not have permanent toilets. These houses receive water erratically from two taps situated in a common space that is shared by all the families. A 2022 study by the UNICEF found that most households in these lines depend on tubewells, but about 15% of the houses have piped water that is supplied by tea estates.
The diet on regular, non-festive days comprises rice, rotis, greens based on their ability, sometimes lentils, and a strong reliance on potatoes and onions as they are cheaper than other vegetables.
“The executive staff members received five times more water per day than the labour lines, while the Managers' bungalows were endowed with 25-35 times more water than the labour lines,” the study notes. Oraon remains hopeful that the conditions in the labour lines will improve in the future, and that she can finally own the house she and her family resides in.
Irrespective of her shift timings, Oraon is up early to prepare the day’s meals, including the lunch that she will carry to her workplace. The diet on regular, non-festive days comprises rice, rotis, greens based on their ability, sometimes lentils, and a strong reliance on potatoes and onions as they are cheaper than other vegetables. Snacks remain elusive owing to the lack of time to prepare them, as does fruit, because of prices.

“We do not have the luxury of time to think about meals. Come rain or shine, we have to fulfil the target to get the full day’s wage. In my case, I am the only one in my family employed in the tea garden. I have to work all six days,” she adds, while grinding white mustard seeds and a ghost chilly to a paste on a grindstone. She also throws a few cloves of garlic onto the stone.
Oraon also chopped up pieces of an elephant apple (Dillenia indica) she foraged while returning from her shift, along with some local ferns and herbs. She starts sauteing them with the paste she prepared. “Foraging is an integral part of our identity. Our grandparents learnt it from their parents, who passed down this knowledge about various edible plants and fruits to us. This produce provides relief to the body. I make sure that my children eat it over all the unhealthy fast food that we find here,” Oraon says as she packs her lunch. She sets off, with her box of chapatis, chillies, onions and the sauteed elephant apple fry.
Also read: Mumbai's mill-era 'khanavals' fuelled a workforce with affordable, homely meals
Tea workers get assigned across different sections of the estate, which could easily be a 2 to 3 km trek through an undulating terrain teeming with surprises. “The company provides us with tarpaulins and umbrellas to wade through these leech-infested gardens for our safety. However, we stay out in the sun or heavy rains, which takes a huge toll on our bodies.” Oraon prays to the tea bush with folded hands, before quickly plucking a handful of leaves from the plant, depositing them in a conical basket. “We are supposed to weigh the produce thrice in the day. Clocking 28 to 30 kg of raw tea leaves every day is not an easy task,” Oraon explains, emptying her basket. She sings a few lines from her favourite song as her hands move deftly through the plants.

By lunch, the sky is overcast. Within minutes, Oraon and others start moving the produce. Carefully avoiding slippery patches of slush and muddy slopes, the workers carry their leaves to the weighing scale under the shed. A few women start filling up their bottles with a red-brown liquid, called ‘chai pani’ or salty water boiled with tea leaves—a practice foisted on tea workers since colonial times, as a means to tackle the serious dehydration they face. The consumption of chai pani over time has been detrimental to tea workers’ health; the excess sodium has resulted in an increase in cases of hypertension and heart and kidney ailments. Additionally, excess tannin–which is a major component of tea–is known to decrease iron absorption; for the many anaemic women in the estate, this salted tea is greatly damaging.
‘Chai pani’ or salty water boiled with tea leaves—a practice foisted on tea workers since colonial times, as a means to tackle the serious dehydration they face. The consumption of excess sodium and tannins has been detrimental to their health.
Although many tea gardens across Assam are supported by globally-aided non-profits working for improved living conditions and better nutrition, such efforts have barely had any impact on the public distribution system that is responsible for providing the workers with food rations once in a week. “We get 1.5 kgs of rice and flour per person, as well as tea leaves as rations. We have to buy vegetables, lentils, oil, sugar and salt from the markets,” Oraon says. Many of these initiatives talk about the importance of consuming protein and fortified foods, while the practice of foraging wild herbs and locally growing fruits remains understated as a source of nutrition.
To observe foraging first hand, we follow Oraon and her comrades, who lead us to a stream that flows swollen from this afternoon’s heavy downpour. She tells us that the freshwater stream also provides the communities with crab and small fish. The women burst into laughter while separating a skunk vine (Paederia foetida) from the undergrowth. “We call it Padra Paat because of its peculiar flatulence-like smell,” she laughs. Across Assam, various communities consider skunk vine as a source of anti-diarrheal properties. Oraon explains that for anyone suffering from stomach ache or indigestion, the medicine is simple: fritters made from skunk vines, mashed into boiled rice.

Within a few metres around the gushing stream, Oraon and others collect ferns, taro leaves, Moringa leaves (Moringa oleifera), Laksa leaf or Vietnamese coriander (Persicaria odorata) and elephant apple. The women relay the health benefits of their harvest–ranging from an antidote to aches and pain, to improving digestion, and acting as effective anti-diabetic medicine.
This traditional knowledge of herbs among the tea workers has also been studied by agricultural scientists from Assam Agricultural University (AAU). In a 2020 study conducted in Dibrugarh district, the researchers counted as many as 20 plants used by tea workers in Assam as medicinal herbs. While pharmacological investigations are yet to be conducted on many of these plants, some of the well-known ones such taro and moringa leaves are considered as superfoods, rich in micronutrients. Yet, Oraon and others caution against eating these plants growing right next to the tea gardens. “We usually do not forage inside the tea gardens because the management uses pesticides and other chemicals to protect the tea. Since the gardens are close to forests and hill slopes, we find our plants there,” Oraon adds
Later, during the lunch break, Oraon and other workers share meals from their ‘tiffin boxes’. “We get about 20-30 minutes to finish lunch. There is enough for everyone as we share our meals, and in between these meals, we poke fun at each other or find a shoulder to lean on when we are upset,” says Oraon. Most workers bring vegetables, ferns and lentils. Oraon eagerly awaits the clock turning 4, when her shift can finally be over.
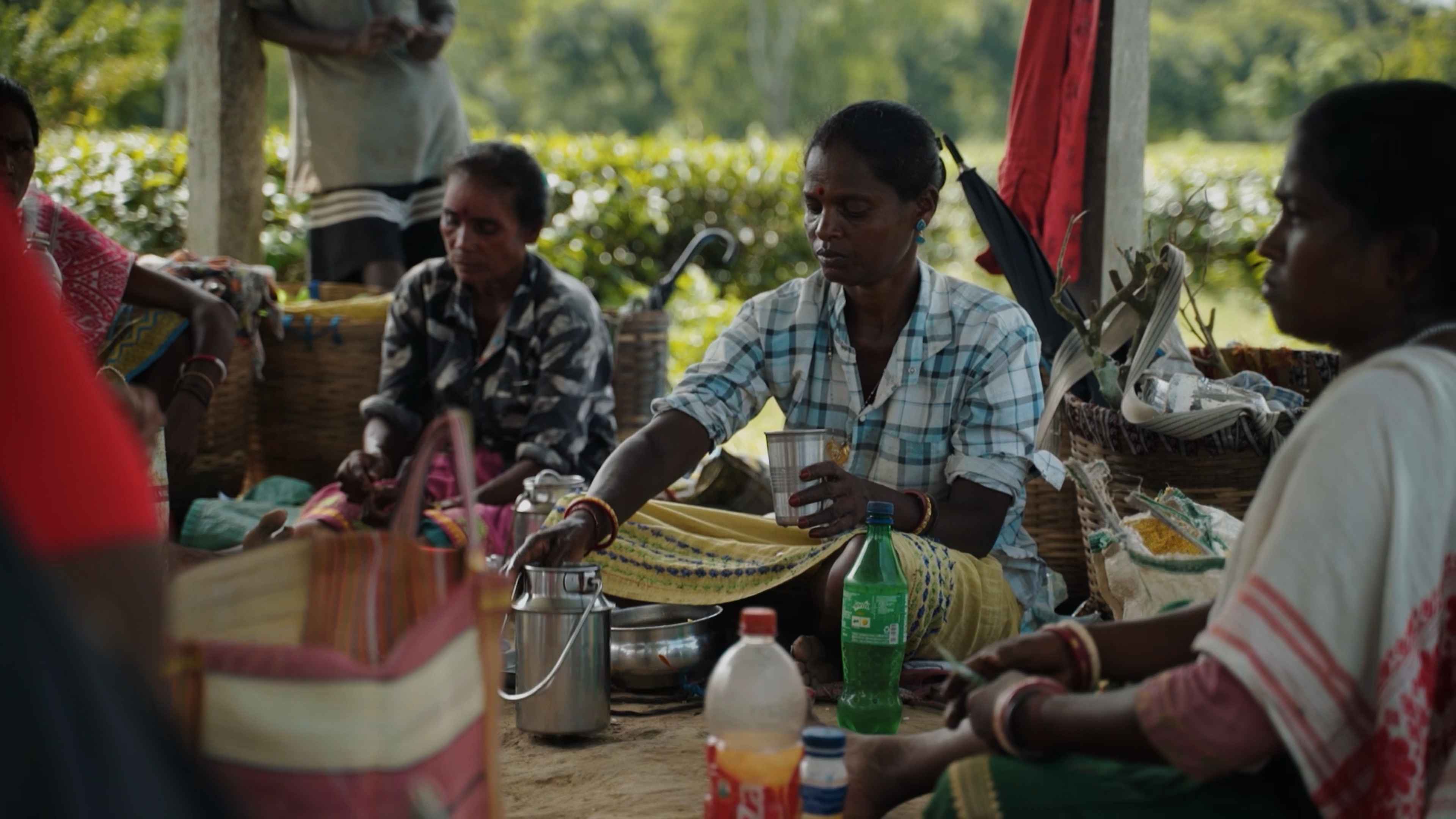
Surrounded by the warmth of the hearth in her home, Oraon explains the core of what sustains her and others in the labour lines. “For us, our job as permanent tea workers is an asset. One of the family members has to be employed in the tea garden so that we get housing provided by the tea company. We are told that now we will be able to own the land and the house. We want the house to our name. It should happen soon,” Oraon hopes.
In the last few years, the Assam tea industry has witnessed a historic revival; after a period of 25 fallow years, Assam noted an increase in its exports by 40 million kg. The losses in production are owed in significant part to factors of climate change. In June of last year, production fell by 12% entirely due to a 50% rainfall deficit in the tea garden regions. Workers and their livelihoods, thus, are also highly vulnerable to these extreme weather conditions. Oraon and thousands like her continue their fight for better wages and rights over their own land–the land that they tamed for the tea industry to thrive.
Also read: Protecting place and power, not people: The trouble with GI tags
Edited by Anushka Mukherjee and Neerja Deodhar
{{quiz}}
Our dinner plates now carry the accumulated chemical burden of decades of systemic lapses, while cleaner, safer food—a basic right—comes at a premium.
Editor's Note: The planet we inherited as children is not the planet we will someday bid goodbye to. The orchestral call of cicadas in the evenings, the coinciding arrival of the monsoon with the start of the school year, and the predictability of natural cycles—things we thought to be unchanging are now at risk. An altered climate, declining biodiversity and warming oceans aren’t distant realities presented in news headlines; they affect us all in seen and unseen ways. In ‘Converging Currents’, marine conservationist and science communicator Phalguni Ranjan explores how the fine threads connecting people and nature are transforming with a changing planet.
As a country where the cuisine morphs every few hundred kilometres, our relationship with food is not merely one of necessity and survival; it transcends an entire spectrum of emotional, cultural, and sensory experiences. Most of us enjoy the rich diversity of flavours that Indian cuisines offer us, but do we know what is really on our plates?
Every bite holds the entire journey of every single thing that it is made of, and made from: the ingredients, soil, water, air, farm animals, decades of chemistry, and centuries of culinary magic and culture—and unfortunately, contaminants. Washing, cooking, and processing can eliminate a significant number of the surface chemicals, impurities, and microbes that come along with our food. However, contaminants like heavy metals, legacy organochlorine pesticides, and veterinary antibiotics can persist in our food despite our best efforts.
This includes the notorious ‘forever chemicals’ per- and polyfluoroalkyl substances (PFAS), which can take hundreds of years to degrade, and whose traces are still found in food globally.
Two chemical properties explain why many of these contaminants stick around in the environment, food, and even in living beings: lipophilicity (being fat-loving) and persistence. Fat-soluble pesticides and industrial chemicals make their way into fatty tissue where they are stored rather than being broken down or excreted. Their molecular structures also resist chemical and microbial breakdown, so they remain intact for years, moving slowly from soil to plant (to animal fat in the case of animal products) to the human diet.
For most of human history, food contamination was accidental and local: smoke residues from fires, naturally occurring plant toxins, spoilage, poor storage, or naturally occurring soil pathogens. In the 19th and 20th centuries, the industrial revolution, rapid urbanisation, mechanised agriculture, and the rise of chemical inputs very quickly altered how food was grown, preserved, processed, and transported.
In the mid- to late-20th century, the Green Revolution brought in high-yield crop varieties and hybrids to boost the agro-economy across the world. Realising this vision required intensive inputs of fertilisers, irrigation, and pesticides, increasing chemical dependence to boost yields. Globally and domestically, food production rose dramatically, but so did the chemical load within agroecosystems, most detrimental of which are persistent organic pollutants (POPs)—pesticides and fertilisers so resistant to degradation that traces exist to date. This includes the notorious ‘forever chemicals’ per- and polyfluoroalkyl substances (PFAS), which can take hundreds of years to degrade, and whose traces are still found in food globally.
Driven by this very need for yield, around the same time, antibiotics were made mainstream in animal husbandry globally, without stringent regulations monitoring their usage. This laid the groundwork for antimicrobial resistance, with drug-resistant bacterial genes now living on in the environment, making it difficult to treat infections.
While high levels of all these contaminants can cause severe acute side effects when consumed, there are also effects that compound over time, silently. Chronic low-level exposure can cause a range of neurological, cardiovascular, developmental, metabolic, and endocrine complications in addition to organ damage, cancers, reproductive dysfunctions, severe allergies, and even affect the brain. Microbial exposure can result in chronic gastro-intestinal disorders, the permanent impacts of which can cause recurring future infections. Pregnant women, the elderly, immuno-compromised people, and children are especially vulnerable, as low-level chronic exposure is typically only detected when there are clear symptoms, sometimes too late.
The concept of food safety addresses the effects of these contaminants on people as they pose a public health challenge. However, the issue of environmental impacts and animal suffering tends to fall through the cracks. Excessive, long-term pesticide use also negatively impacts the environment in general, including soil fertility and quality. Through runoff and waterways, these chemicals ultimately reach water bodies and the oceans where they affect aquatic life. Heavy metals and legacy pesticides now pass on from mothers to young ones with toxic effects, fish show gill deformities, reproductive cycles are disrupted, and navigation and behaviour are impacted.
Even tiny concentrations can disrupt how bees navigate, feed, and move; and how birds reproduce, communicate, and grow. Most pesticides also cause endocrine (hormonal) issues, resulting in reproductive and physical deformities in animals, from small frogs to large polar bears.
Also read: Bugging out: Why declining insect populations in India spell doom for agriculture
The entry of contaminants into food arises at multiple points in the farm produce-to-dining-table continuum. Airborne emissions settle onto farmlands, contaminated water irrigates crops, residues persist in soil year after year. Plants absorb what is available, animals eat the plants, and we humans consume both.
Soil and water are the most direct routes for contaminants to enter the food chain. Industrial emissions, mining, fertilisers, pesticides, mismanaged sewage, and wastewater release lead, cadmium, arsenic and other heavy metals into agricultural soils, waterways, and the air. Plants take up a share of these heavy metals through their roots, and leafy vegetables often show the highest concentrations. Pesticide sprays settle on leaves and fruits, and some systemic insecticides move throughout a plant’s body to deposit in edible tissues.
Antibiotics given to livestock can leave residues in meat, eggs, and milk when withdrawal periods—mandatory waiting time after administering a drug before animal produce is safe for consumption—are not observed. In principle, regulatory checks should prevent significant residues, but lapses in compliance or weak enforcement mean residues reach the consumer’s plate.

The absorption of chemicals and heavy metals into fats results in biomagnification as the food web progresses. Plants and small animals absorb them, larger animals feeding on them accumulate higher levels, and we ingest a disproportionately larger share when we consume fatty meats, plants (nuts, seeds), or dairy. The same happens in edible fish and shellfish which accumulate contaminants released into marine and freshwater environments; these typically include mercury and toxic persistent industrial chemicals such as polychlorinated biphenyls and perfluoroalkyl substances.
Food processing and packaging can also introduce contaminants along the chain. Milling rice, refining oils, or drying spices may reduce contaminant level, but can inadvertently introduce others. Contact with plastic packaging introduces compounds such as bisphenols, phthalates, microplastics, and perfluorinated chemicals into food, especially in canned or high-fat products. These substances, much like the other contaminants, are widely associated with neurological, hormonal, cardiac, respiratory, and metabolic side effects.
Heavy metals such as lead, cadmium, mercury, and arsenic are among the most concerning and common food contaminants because they accumulate without degrading. However, their presence in food can be due to the geology of the region (unavoidable) and industrial activity (uncontrolled), rather than agricultural practice alone.
Plants and small animals absorb them, larger animals feeding on them accumulate higher levels, and we ingest a disproportionately larger share when we consume fatty meats, plants (nuts, seeds), or dairy.
Arsenic, for example, occurs naturally in certain soils and groundwater. High levels in groundwater have been reported from several Indian states including West Bengal, Bihar and Uttar Pradesh, while traces have also been found in rice grown under flooded conditions, making it a significant concern in Southeast Asia.
Industrial processes such as mining, smelting, and fossil fuel combustion, combined with sewage sludge being used as manure, release toxic metals. Once in the soil, they are taken up by leafy vegetables, root crops, and grains, or enter aquatic food webs, ultimately accumulating in foods we eat. Urban and peri-urban agriculture is equally vulnerable due to contamination from traffic emissions, wastewater mismanagement, and industrial emissions.
Also read: Home gardens enrich the soul. Can they improve urban biodiversity too?
Pesticides and herbicides improve yields and reduce pest damage, but toxic residues persist in the crop despite processing. During the Green Revolution, agriculture relied heavily on organochlorines like DDT, lindane, and dieldrin, a group of pesticides so persistent that they remain detectable in soils and sediments even now, decades after their ban. Many of these pesticides were recognised later—perhaps too late—as toxic and potentially carcinogenic to humans, wildlife, or both.
DDT was also, until recently, hailed and abused as a miracle against vector-borne diseases like Malaria. India, once the largest user of the chemical, now remains its sole manufacturer since 2008, even as authorities talk about phase-out plans.
Today, pesticide chemistry has shifted to marginally better formulations. While many are less ‘immortal’, they are still associated with neurodevelopmental, organ-related, metabolic, hormonal, developmental deformities, and other side effects associated with chronic low-level exposure. Farmers and their families, with the highest exposure risks, tend to suffer disproportionately more.

The indiscriminate use of antibiotics in livestock and poultry is a hotly debated topic. Shifting diets and increasing demand for animal protein have driven up production; resultantly, the cramped and unhygienic conditions of large-scale poultry farms often result in disease outbreaks. Antibiotics and anti-parasitic drugs are frequently administered not only to treat disease but preventatively—a practice that has been critiqued extensively, but one that continues, regardless.
Antibiotics and anti-parasitic drugs are frequently administered not only to treat disease but preventatively—a practice that has been critiqued extensively, but one that continues, regardless.
While some countries tightly regulate these practices, drug residues can still find their way into meat, milk, and eggs. Residues in food, even below regulatory limits, can selectively make some pathogens immune to them, reducing the effectiveness of life-saving medicines, as is being increasingly observed in emerging strains of drug-resistant diseases. The implications extend beyond just exposure: the broader global public health crisis of antimicrobial resistance (AMR) is a cause for concern, and now affects wildlife as well, making it more difficult to treat infections.
From smallholder farms to intensive dairy operations, India’s food system is vast and diverse, and the pathways for contaminants are just as numerous and unevenly distributed. Traces of heavy metals like lead, cadmium, and iron in vegetables and leafy greens are regularly reported from multiple parts of the country, especially industrialised and urbanised regions, at concentrations far exceeding permissible levels.
A very recent analysis of vegetable and soil samples from Bengaluru found toxic levels of lead in amounts that are 5-20 times higher than permissible limits. 26% of the vegetable samples—some that claimed to be organic—and over 85% of the soil samples from source farms were found to be significantly contaminated.
Whether it is recent reports of potentially carcinogenic nitrofurans detected in eggs, coliform bacteria in milk and curd, fake or analogue paneer doing the rounds, or even adulterated honey, the problem is not new.
A seven-year-long observation (2013 to 2020) of vegetables across Northern and Western India found residues of 56 pesticides in 40% of the vegetable samples including capsicum, brinjal, gourds, and tomato. While the levels mostly fall within permissible non-toxic limits, and a similar trend is reflected internationally, chronic low-level exposure remains a critical concern.
Organochlorine pesticides have been routinely detected in ghee and butter for decades now, while adulteration of milk, spices and condiments have been a widely accepted reality for just as long. Despite reiterated bans and regulations, antibiotic residues in meat and poultry are also routinely detected at levels especially harmful for children.

Whether it is recent reports of potentially carcinogenic nitrofurans detected in eggs, coliform bacteria in milk and curd, fake or analogue paneer doing the rounds, or even adulterated honey, the problem is not new.
The issue lies not with our dietary choices of vegetables, dairy, or meat, but rather, with the regulatory and processing chains. While it is not easy to feed the world’s most populous nation, the kind of economic forces that drive our markets push for quantity over quality, and profits over safety. The global story mirrors India’s, with legacy pollutants, antibiotic residues, and resistance genes sparking concerns. However, that cannot be an excuse to brush domestic issues under the carpet in what is most certainly a systemic failure.
For consumers, transparency through clear labelling and traceability can empower informed choices. However, addressing food contamination requires shifting focus from end-point testing to upstream prevention and improving safety standards and regulation. Quality monitoring needs to become routine rather than reactive. For example, the Food Safety and Standards Authority of India (FSSAI) was recently spurred into action after reports of contaminants in eggs surfaced, prompting a sudden flurry of widespread and deeper testing of eggs while routine monitoring seems to be largely absent.
Improving the processing chain and plugging in the gaps by integrating robust data and technology into food systems, and improving screening, sampling, reporting, accountability, and emergency responses are other areas that need focus. However, the driving of holistic solutions on ground also needs to involve stakeholder collaborations for widespread, equitable, and sustainable adoption.
The bottom line is that modern agriculture, evolving technology, better awareness, and food processing have not eliminated the problem of chemical residues. In fact, unfortunately, some of the sources arise from compulsions to meet demands: intensive agriculture prioritises high yields and protection from pests and infections; untreated or partially treated wastewater is the only alternative in water-scarce regions.
Products from multiple big, ‘trusted’ brands continue to fail independent third-party quality testing, and these brands—and regulatory bodies—only spur into action to address claims instead of proactively stepping up.
What is equally concerning is the socio-economic divide in access to ‘better’ and perhaps ‘safer’.
What should be working and evolving is the regulatory framework and accountability.
It is widely accepted that screening and enforcement is uneven, not just in informal markets and among smallholder farmers who may lack access to safe inputs or proper training, but among the big, moneyed players. As a result, chemicals and drugs meant to protect crops and animals continue to flow into diets, putting millions at risk.
In some ways, these contaminants are not merely chemical intrusions, they are indicators of how we have shaped our environment in the pursuit of ‘more’.
What is equally concerning is the socio-economic divide in access to ‘better’ and perhaps ‘safer’. Foods with a guarantee of being cleaner, safer, and better regulated are now priced at a premium for what should be a basic standard. How much of India’s population would understand contamination or product recalls in the first place? How many are able to pay double for a safer label to feed a family, or pay a premium for a tub of curd when the low cost alternatives carry contaminants?
I cannot think of any foolproof solutions to this, nor do I know if the ‘safer’ choices I’m inclined to pick are even safe—trust is a precious yet fragile thing brands tend to exploit. I do recognise that I have the immense privilege of choice, and access to ‘better’. But what about those who do not? When did it become okay to pay a hefty premium for cleaner, safer, healthier food—something that should be a given?
Also read: Climate change in my cup: Why India’s cocoa and coffee production is at risk
Cover art by Pratik Bhide
{{quiz}}

Water bodies help these hardy farm animals regulate their body temperatures in India’s hot summers
With crescent horns and a love for ponds, the domesticated water buffalo—Bubalus bubalis—is a familiar presence across India’s farms and floodplains. Yet, despite its ubiquity in Indian agriculture, one of its most distinctive traits is also the most misunderstood: its unwavering devotion to water.
Buffaloes are often seen submerged in village tanks, irrigation canals, or muddy pits, eyes half-closed in apparent bliss. This is not laziness, nor mere habit. It is physiology: unlike cattle, buffaloes are built for heat differently—and that difference has shaped where they thrive, how they are managed, and why water access is central to their well-being.
India’s summer temperatures regularly cross 40°C. For large-bodied mammals, heat dissipation becomes a daily challenge. All warm-blooded animals must maintain a stable internal temperature; when environmental heat exceeds body temperature, the animal must actively shed heat or risk stress, reduced productivity, and even death.
Buffaloes face a structural disadvantage here. Compared to many cattle breeds, they have darker skin, sparse hair, and relatively fewer functional sweat glands. Dark skin absorbs more solar radiation. Sparse hair means lesser insulation from direct sunlight. And limited sweating reduces evaporative cooling—the primary method by which many mammals lower body temperature.
This is where water comes into the picture. When a buffalo submerges itself in water or mud, it uses conductive and evaporative cooling to regulate heat. Water, being cooler than the animal’s body, draws heat away through direct contact. Mud adds another layer of protection: as it dries on the skin, it continues to cool through slow evaporation while also forming a barrier against biting insects.
In effect, wallowing is a substitute for sweating and an adaptive behaviour. Buffaloes that lack access to wallowing sites show clear signs of heat stress: increased respiratory rate, reduced feed intake, lower milk yield, and altered reproductive cycles. Dairy studies have repeatedly demonstrated that buffalo milk production drops significantly during peak heat unless adequate cooling systems—ponds, showers, sprinklers, or shaded housing—are provided.
In effect, wallowing is a substitute for sweating and an adaptive behaviour.
This reliance on water also explains the geographical distribution of buffalo populations, which flourish in river basins, delta regions, and areas with irrigation infrastructure. Historically, their association with paddy cultivation in wetland ecosystems made ecological sense: fields, canals, and ponds doubled as thermoregulatory resources.
Also read: Water buffaloes: A historical look into their role in agriculture
At first glance, buffaloes and cattle appear similar enough to share identical coping mechanisms. But cattle (genus Bos) possess a higher density of active sweat glands, making sweating a more effective cooling strategy. Buffaloes, in contrast, have fewer sweat glands and lower sweating rates.
Research comparing the two shows that under equivalent heat stress, buffaloes exhibit higher skin temperatures, greater reliance on increased respiration (exhibited through panting) and a stronger behavioural drive to seek water. This reliance on water is not a liability. In fact, buffaloes are remarkably resilient in tropical climates where sweating alone would be inefficient. High humidity reduces the effectiveness of evaporative cooling. In such environments, access to water bodies for immersion can be more efficient than surface evaporation alone, and helps these creatures stay healthy.
Also read: Chicken manure is clucking good
The image of a buffalo immersed in a pond is not a pastoral cliché. Water is not just something the buffalo loves to paddle in. It has evolved alongside India’s wetlands, floodplains, and monsoon cycles. It is part of what allows this animal to endure Indian summers—and continue to power one of the world’s largest dairy economies.
{{quiz}}

Safe spaces, outlets for creativity and curiosity, and a connection to the Earth—nature can mean many things to kids
Editor’s note: To know Rama Ranee is to learn about the power of regenerative practices. The yoga therapist, author and biodynamic farmer spent three decades restoring land in Karnataka, envisioning it as a forest farm in harmony with nature. In ‘The Anemane Dispatch’, a monthly column, she shares tales from the fields, reflections on the realities of farming in an unusual terrain, and stories about local ecology gathered through observation, bird watching—and being.
To Ananda, our farmhand Rajappa’s son, herding cows in the forest was familiar territory—like it was for many others from villages bordering Karnataka’s Bannerghatta National Park. Trees were sparse in the area, but there were grasses and shrubs. When he was about 12, he set off with his neighbours to graze the family’s 20 heads of cattle. Like a bolt from the blue, a leopard leapt at one of the heifers, latching on to the jugular vein. Her mother, a tall cow with formidable horns, charged at the leopard and tossed it aside. Ananda and companions warded off further attacks, screaming and shouting on top of their voices.
While all the other cows surrounded the injured calf, a jersey sounded the alarm, bellowing till the menfolk came from the fields. The leopard escaped, but the calf did not survive. Ananda continued to herd during his spare time from school—where encounters with the wild were commonplace: a sleeping python, a sunbathing crocodile, and all the learning that came with them.
Such a job appears to be fraught with risks. By necessity, children and women are often tasked with herding, but it is a community effort with many herders getting together for security and convenience, offering unique learning opportunities. The knowledge of medicinal and edible wild plants was passed down to the youth from more experienced herders, who were also foragers, like Ananda’s own grandmother.
Ananda does not herd cows anymore; he works at a mall, but the chord that connects him with the land is still visible, though frayed. Such connections with nature are becoming rare as we hurtle towards rapid urbanisation—a real, palpable loss because children who bond with nature are likely to value and care for it. Enjoying nature is not the same as living in connection with it. There is no objectification in connection, only a certainty of belonging—a harmony that is felt.
Enjoying nature is not the same as living in connection with it.
Also read: In forest bathing, an invitation to heal by being one with nature
The development of a child is a marvel. An infant who seemed no different to others, who goes through the same patterns of movement and stages of growth, has a unique personality by the age of 3! What we witness is the unfolding of inner potential, from birth to adulthood, through a process of neurological maturation. The early years are crucial because organs like the brain and nervous system are still undergoing changes and adaptations, transforming well into adolescence.
Though genetics are a major determinant, the environment within which a child is raised provides a powerful stimulus for growth. The home environment influences major domains of development, from the linguistic and motor, to the cognitive and socio-behavioural functions. A connectedness to nature during childhood has positive effects on kids’ physical and psychological health and well-being. Conversely, a degradation of nature could have a negative effect.
Nature Deficit Disorder (NDD), a term coined by journalist and author Richard Louv, refers to the combined psychological, physical, and cognitive costs that children in the vulnerable years of development suffer due to alienation from nature. It lists diminished use of the senses, difficulties with attention, and higher rates of physical and emotional illnesses such as depression as possible consequences. NDD can affect individuals as well as families and entire communities.
Growing physical inactivity in children has been identified as a serious public health concern, as it has multiple ramifications. Increased obesity, metabolic risks, cardiovascular diseases, and Type 2 diabetes are on the rise, especially among school-going adolescent children. Inadequate access to playgrounds and green spaces in urban areas, compounded by sedentary habits, contribute to these conditions. Studies support the theory that green spaces enhance both physical activity and emotional well-being.
The introduction to a wider world outside the home begins with an early education system: Kindergarten, a half-way house between home and school. ‘Kinder-garden’ as it is colloquially called here, was meant to be a ‘garden of children’, designed to nurture them much like plants are by skilled gardeners. Established by the German educator Friedrich Fröbel in 1840 in Prussia, it prioritised interactions with nature, including growing plants and observing them, to introduce young minds to the unity of and diversity within the natural world.
Here, learning happens through guided play, songs, and artistic activities, amid peers and friendly adults in a safe and beautiful environment. The process allows the child’s own faculties to unfurl like a plant, as nature intended, prompted by an inner impulse. The changes that occur are biological and social, impacting the child’s ability to act in the world and live an enriched, harmonious life.

Fröbel’s proposition is an idyllic situation, a far cry from reality. In most Indian cities, including smaller ones, education has engulfed the child even before his or her faculties are ready for academic learning. Schooling starts at 2+ years in institutions where ‘gardens’ have given way to enclosed spaces, with undue emphasis on competitive accomplishments; where structured instruction is the norm, and creativity is confined to art sessions and mandatory staged performances on ‘school annual day’.
The process allows the child’s own faculties to unfurl like a plant, as nature intended, prompted by an inner impulse.
To facilitate learning within nature in cities is not impossible. A daily visit to a park or a little terrace, or backyard gardening with the added attraction of birding, could brighten the child’s day—and perhaps his or her future, too. The experience with my own sons at Anemane taught me invaluable lessons in early education which I later applied in my work as a therapist with other children.
Also read: Friends of the soil: A farmer's key allies hide backstage and underground
There was a boy of four
Who was out of the door
In the garden or on the stair
Lighting fire, catching frogs, everywhere
But never, ever, in his chair.
There was a Rose Apple tree in the backyard which hosted squirrels, mynas, bats, and a family of cats—a happening place! I told my son that we’d be doing something very exciting in its shade, excavating a pit like archaeologists to discover ancient buried things. The branch displayed a board that said ‘Laboratree.’ The excavation yielded tea cup shards, rusty shaving blades, a pen, and many other things which were carefully removed and observed. Experiments that rivalled an alchemist’s carried on, as fluids were boiled, a magnifying glass was put to good use to singe leaves or watch worms, and the seeds of a precious Ashoka tree were sown in repurposed cans. The seedlings were strong and deep-rooted.

When he entered school, he gifted them to the garden on campus. He found his roots, too, in the forests enveloping the school, exploring, working with his hands, undertaking carpentry, sculpting under the canopies, and discovering a rhythm that resonated in the dholak—free-flowing, but centered in an intuitive way.
Now, three decades later, he reflected upon the most significant learning of that phase: a sense of security and belonging in wild spaces, keen senses, self-reliance, fearlessness, and physical endurance. These are attributes which shaped his personality.
Imagination is the foundation for original, independent thought, and natural environments contain boundless opportunities for the stimulation of a child’s curiosity and channelling of his or her creativity. The sounds, sights and feel of things, such as the drifting of clouds, the flow of water, and wind blowing through the branches, widen the young mind. When experiences are absorbed and internalised, they become the building blocks of knowledge.
A world of make-believe offers infinite possibilities. When my younger son was a little over 2, we went to the park to play. With watchful eyes, he saw a row of tall, thin trees, swaying in the breeze. One day he found smooth, oval pellets on the ground. With wonderment he picked one up, cradling it in the palm of his hand. Was it a bug or a seed? It was a ‘Tossy-bug’—a name he gave to the seed, which he believed was also a bug, interchangeable, animate. From then on, ‘Tossy-bugs’ were everywhere: in his colourful paintings, conversations and fantasies.
The sounds, sights and feel of things, such as the drifting of clouds, the flow of water, and wind blowing through the branches, widen the young mind. When experiences are absorbed and internalised, they become the building blocks of knowledge.
Today, he is an environmental management professional who says that the seeds entirely altered his mindset. “Even now when I doodle, I only doodle Tossy-bugs, birds, and hills. It opens up an entire alternate dimension of nature, and shapes one’s imagination and ability to not feel alone or afraid while on one's own in forests, because it feels like there are friends and friendly beings all around.”
Young children aged 6 to 12 tend to be more deeply connected with nature, but it is never too early to introduce a child to its mysteries. On a warm day, as birds leapt out of the golden yellow grass, Sriyu dangled his feet in his carrier against his mother’s belly while we walked listening to bird songs. All of 16 months and yet to articulate with words, he gesticulated, directing our attention towards the object of his interest. Released from his carrier, he toddled—looking for twigs, leaves, and anything else he could find, oblivious to scratches and falls. He finally squatted at a stone, placing his findings on it.

A seasoned traveler, he was happy to narrate his experiences through sounds–the roar of a tiger, a tigress with cubs (the two sounds were distinct), the family sighted in a tiger sanctuary, birds, and our cow. His parents believe in bringing him up as close to nature as possible, having themselves benefitted from childhoods amid rambling gardens with large trees and forests. Owing to professional compulsions, they are based in the city, but their hearts remain in the wilderness. Since the age of four months, their baby has witnessed forests, mountains, and the sea. When he met the full-bloomed version of a rare flower and smiled at it, his communion with nature had begun.
What Sriyu’s parents wish the most for their child is clean air, pure water, a green Earth and a life free of pollution. In their view, affluence alone does not ensure happiness or the ability to deal with the vicissitudes of life. Proximity to nature builds resilience and detoxes oneself.
They can already notice the impact of nature on their child, who enjoys long, restful, and timely sleep unlike the disturbed sleep rhythms in the city. They also see how he engages with the surroundings, and the building of his acute observation skills. At Anemane, when Sriyu’s gaze followed the stream of water dazzling in the sunlight, I felt in my bones a hope that all is not lost for the planet.
Also read: Farming under the elephant's nose: Lessons in crop choices
For neurodivergent children, especially those on the autism spectrum, natural environments are therapeutic. The gentle sensory stimuli, a rough terrain and the demands and challenges of outdoor and farm life offer opportunities for movement and coordination with their own rhythmic patterns—quite different to a controlled, manmade environment. Internal bodily rhythms such as sleep and wakefulness are regulated, as they are influenced by nature’s forces like the sun and moon.
Gradually, the foundation for the inner core that we call the self or individual is built from where the child feels secure enough to step out into the world. This could well be the preparation for formal learning and social engagement that current systems do not address.
Aadir, a neurodivergent child and my yoga student of eight years, has matured into a sensitive adolescent who attends a regular school, but loves gardening and music, too. His mother recalls that he loved playing with water and mud. Then he began to take an interest in insects and birds, which elicited emotional responses—a difficult thing for him. Baby fish moved him to pity, and the sea evoked awe as if he is touching ‘the ancient water of Earth’.

Gardening vegetables like okra, onion, and pumpkin engaged his hands and gave him a sense of responsibility. “His feelings have gradually developed over the years, and we are blessed to have the garden. I see a deepening of interest in the living environment. I believe it marks a significant turn in his development as an individual… Every time he spends time connecting with nature, I find him a little more connected to himself. He is more responsive to our emotions, too,” says his mother. Other vital changes that nature induced in Aadir were calmness, relaxation, and sleep regulation.
The way to save the environment is to rescue the child and offer him or her the woods—and these woods could be a garden in the city, too!
Not everyone who experiences this connectedness probably depends on it for a living, or wishes to become a professional in related fields. However, the bond will go on to determine many life choices—especially those that compel one to protect nature and nurture it. The way to save the environment is to rescue the child and offer him or her the woods—and these woods could be a garden in the city, too!
Artwork by Khyati K
{{quiz}}

Making up nearly 50% of the country’s milk production, this domesticated animal has called the Indian subcontinent its home since ancient times
A sturdy broad-shouldered build, nearly six feet tall, a greyish black coat, two formidable horns, steady two-toed hooves, and an endearing affinity for water. The domesticated water buffalo—called bhains in Hindi, gedhe in Telugu, erumai in Tamil—is instantly recognisable across the country. The scientific community delightfully christens it Bubalus bubalis.
Wild water buffaloes are native to the Indian subcontinent. Around 6,000 years ago, some of them were domesticated in northwestern India. They evolved into present-day 'river buffaloes' (B. bubalis bubalis), reared primarily for milk production. A couple thousand years later, along the Indo-China border, the same species evolved into a different domesticated animal: the swamp buffalo (B. bubalis kerabau). These buffaloes love muddy swamps and make for great draught (or working) animals for ploughing paddy fields. Many Southeast Asian countries continue to rely on swamp buffaloes.
Why do we go through the trouble of calling them ‘water buffaloes’ when we could just say ‘buffaloes’? One might first assume that their name stems from their tendency to wallow in water on hot summer days. But by that logic, why are crocodiles not called water crocodiles?
The truth, like the water that buffaloes tend to wallow in, is muddied—by history. The buffaloes Indians know and love are not the only ‘true’ buffaloes. Water buffaloes have a land-loving (and somewhat violent) wild cousin called the African buffalo (Syncerus caffer). To add to the confusion, Americans (misled by some 17th century French fur-trappers) mix up bison (which are native to the region) and buffaloes.
Despite sharing the same colloquial name, these are three distinct species and fairly easy to distinguish from one another. African and Asian buffaloes belong to one lineage within the bovine subfamily (Bovinae), while bison—though related—form a separate branch. Both types of true buffaloes prefer the clean-shaven look, while bison are easily identified by their beard. The African buffalo and water buffaloes are most easily differentiated by their horns: if the horns emerge directly from the head in a crescent shape, you are looking at a water buffalo. If the horns are fused to a shield on the buffalo's head (called a boss), then that’s an African buffalo!

Also read: Mumbai’s Nagori dairies are a living archive of milk, migration—and memory
Very early into its domestication journey, the river buffalo discovered its penchant for travelling. The Arab conquests of Egypt and Italy introduced river buffaloes there as early as the 8th century. Their extensive travel led to widespread cross-breeding and a high genetic diversity: there are over 120 recognised breeds of the river buffalo.
It has been sought after because both its milk and its meat are considered nutritionally superior to that of cattle: the fat content of buffalo milk is nearly twice as much as cow milk, and its total solids are nearly 30% higher. This has made it a preferred choice for many countries invested in dairy farming and making processed products from buffalo milk. Over the past century, many of these river buffaloes have also been crossbred and selected specially to give more and better milk, notably in Bulgaria and Italy. The milk of the Italian Mediterranean buffalo is used to make the authentic mozzarella cheese in the Campania and Lazio regions of Italy.
Back home, the cow tends to outshine the buffalo in the public eye. But India remains the water buffalo’s first home. The animal’s presence is firmly etched in both history and mythology. Seals from Harappa, the walls of the Ajanta, folk tales in Jain, Hindu, and Buddhist mythology all make for colourful documentation of our history with the animal.
India continues to house the largest water buffalo population—over 11 crore buffaloes according to FAO 2024 data. In terms of milk production, too, buffaloes nearly equal cows, accounting for almost 44% of the country’s milk production. It would be fair to say then, that these creatures are the invisible engines of one of the largest dairy economies in the world.
The buffalo may not look like the focus of farms, but if you observe closely, you’ll see an animal that is treasured and adored for existing in our landscape.
Also read:The science behind high-protein milk: How it differs from whey powder
{{quiz}}

The Padma Shri awardee’s success is fuelled by tribal agricultural wisdom and an ability to win over sceptics
Editor's note: Every farmer who tills the land is an inextricable part of the Indian agriculture story. Some challenge convention, others uplift their less privileged peers, others still courageously pave the way for a more organic, sustainable future. All of them feed the country. In this series, the Good Food Movement highlights the lives and careers of pioneers in Indian agriculture—cultivators, seed preservers, collective organisers and entrepreneurs.
In the heart of the Sahyadri hills, the monsoon paints the landscape in countless shades of green. On the slopes of Kombhalne in Maharashtra’s Ahilyanagar district (formerly known as Ahmednagar), tucked deep in the mountain range, women move in unison through the afternoon. Their hands sink rhythmically into the muddy red earth as they plant tender rice saplings. A short walk away, Rahibai Soma Popere steps into her modest but well-structured seed bank, where rows of clay pots filled with native seeds lie in wait like quiet sentinels—each one a vessel of resilience and memory.
When she opens the bank’s doors, sparrows fly in. This scene is a familiar one for Popere—one marked by mistakes made and lessons learnt. “Pakshyala kalta kay khayach ani kay nahi. Manasala ekta dnyan asun pan tyala he samajlele nahi.” (Birds instinctively know what is good for them, but humans, despite all our knowledge, fail to understand).

She recounts her experience of planting an indigenous variety of bajra or pearl millet. For the first three years, the crop grew well. Birds arrived at the fields and pecked at it, showing some interest in the grain. By the fourth year, however, something remarkable happened: flocks of birds descended onto the field and devoured the entire crop. Strikingly, they left the surrounding bajra fields, sown with modern hybrid grains, untouched. The birds had a clear preference; they didn’t care for bajra that was harder, bitter, and far less nutritious. The produce from the indigenous seeds, on the other hand, was naturally sweet and soft. “We would crush the grain in our palms and eat it fresh,” she recalls.
And yet, deviating from traditional wisdom, farmers in Kombhalne stopped growing this variety over time as they chased profit and yield. Popere’s own access to the indigenous seeds ended with the birds’ feast. As she gently lifts a pot and scoops a handful of val or ghevda (hyacinth) beans preserved in ash at the seed bank, she reflects on the deeper meaning of this loss. “These are not just seeds,” she says. “They are the heritage of generations, safeguarded for those yet to come.”
Now in her early sixties and affectionately known as ‘Beej Mata’ (Seed Mother), Popere has earned global recognition for her remarkable work in conserving indigenous seeds and wild vegetables.
She was born into the Mahadev Koli tribe and inherited farming as part of community traditions. Hers was a childhood marked by hardship: At the age of seven, she lost her mother. The fifth of nine children, she grew up in a household weighed down by poverty, and was forced to shoulder responsibilities early on—caring for siblings, managing household chores, and tending to the family’s cattle. She didn’t have a chance to formally learn how to read or write. At 12, she was married, stepping into an even harsher life. Within the home of her in-laws, she had neither authority nor respect. Made to live in the cattle shed, she even gave birth to all four of her children there.
She preserved them with great care, storing the seeds in earthen pots layered with ash and sealing the pots with cow dung, a traditional method that the Mahadev Kolis have followed for generations.
“After childbirth, we would return to work the very next day. By the seventh day, we were already back in the fields, with our newborns tied to our backs,” she says. In the 1990s, women in her village were nourished not with semolina or dry fruits, but rather with the Dhavul variety of rice and bhagar (barnyard millet), a highly nutritious grain. These dishes alone gave them the strength to recover, she says. But over time, the millet disappeared from people’s diets.
As a child who grew up watching her community in Kombhalne set aside a portion of their harvest to use as seeds the next season, Popere noticed changes taking place in local agriculture. She distinctly remembers how the wave of hybrid seeds spread across her village in the late 1990s and early 2000s, and eventually arrived at her doorstep. Like many Indian farmers, Popere, too, adopted hybrid crops.
The next few years brought frequent illness to her family, prompting the farmer to see the connection between hybrid produce and health: nutrition was being compromised, and a heavy reliance on chemical fertilisers only made matters worse. “That day (in the early 2010s), I decided we would no longer eat hybrid vegetables and grains in our home. I chose to return to our traditional seeds,” she says with resolve.
At first, her family resisted this decision, as they remained sceptical about whether native varieties would match up to the yield and profits delivered by their hybrid counterparts. But Popere convinced them, over and over, until they finally agreed and began supporting her effort.
Practicing rainfed farming, she has been cultivating native vegetables such as bitter gourd, ridge gourd, sponge gourd, native okra (kate bhendi), cluster beans, and leafy greens like spinach, fenugreek, and dill—grown mainly for its seed in the winter. She also grows numerous varieties of rice such as Ambemohar, Kolpi and Raibhog; numerous types of cowpeas; and 18 types of hyacinth.

Thus began Popere’s endeavour to gather indigenous seeds—beginning with her own home, as well as from her relatives’ and fellow villagers’. She preserved them with great care, storing the seeds in earthen pots layered with ash and sealing the pots with cow dung, a traditional method that the Mahadev Kolis have followed for generations. Seeds stored in ash remain viable for sowing for up to three years, and for consumption, they last as long as ten years, she explains with pride.
After its conception in the mid-2010s, the bank operated from a small mud room on Popere’s farm, where there was no electricity or water connection, and where no more than ten people could stand inside at once. Pots and glass bottles aside, some local beans were strung on ropes, owing to the paucity of space. The bank has lived many lives over the years, expanding in its scope but never straying from Popere’s vision. To her, the challenge in running this bank, which has caught the eye of NGOs as well as the local government, is not logistical or financial, but rather to do with the seeds themselves and their selection.
Also read: How Jayshree Vencatesan got Chennai to finally care for its wetlands
In their village, farming is entirely reliant on the monsoon. The Nilwande Dam towers nearby, but its water rarely reaches the fields. Wells dry up by summer, and even drinking water becomes scarce. After monsoon, most villagers migrate for work; Popere, whose landholding measures 7 acres, would travel across Ahilyanagar, too, to cut sugarcane.
Yet she continued to nurture dreams: she wanted every home to have at least one fruit tree for its children. To this end, she started raising saplings, of papaya, guava, and custard apple among other fruits at home, handing them out to women’s self-help groups or during village festivals. “Once, I raised a nursery of 3,500 blueberry plants,” she laughs. “Everyone thought I was crazy.” Soon after, Pune-based BAIF Development Research Foundation, a rural development NGO, got in touch with her and purchased the entire lot. “I earned Rs. 10,000 on that day—the first time I’d ever seen so much money,” she says with wonder.
Among millets, Popere’s collection featured 12 varieties of finger millet (nagali), both red and white, and two varieties of little millet (varai)—Garvi and Halvi.
Through BAIF’s Community-led Agrobiodiversity Conservation programme, Popere’s effort to save seeds finally found a larger platform. When the BAIF team visited her home, they were astonished to see 125 varieties carefully stored—many rare, some on the verge of extinction. Among them were 16 rice varieties, including Raibhog, Jirwel, Ambemohar, Warangal, and Kalbhat. Of the 28 types of hyacinth beans known locally, Popere had preserved 18, distinguished by their size and colour—green, white, wine, black, long, or short, sweet, or bitter. These varieties continue bearing fruit for up to three years. She also conserved four types of cowpeas. Among millets, Popere’s collection featured 12 varieties of finger millet (nagali), both red and white, and two varieties of little millet (varai)—Garvi and Halvi.
“Until then, my seed conservation and organic farming work was limited to my home,” Popere says. “But with BAIF’s guidance, I began cultivating different varieties solely for conservation.”

Also read: In Leh’s harsh terrain, farmer Urgain Phuntsog is a true ‘mitti ka aadmi’
Popere’s next challenge was persuading Kombhalne’s women about the dangers of using hybrid seeds and the urgency of her work. Few were willing to listen.
The conservation of native seeds is possible only when communities share traditional knowledge. With this objective, BAIF began setting up community seed banks in Maharashtra’s villages to build this collective effort. In 2017, amid interventions in the tribal block of Akole, BAIF and Popere established one such bank in the courtyard beside her home, under the Kalsubai Parisar Biyane Sanvardhan Samajik Sanstha, a community-led seed savers’ group.

The bank began with a simple rule: borrow one kilo of seed, return two after harvest. Through this system, Popere built a network of over 3,500 farmers, training them in seed selection until they could maintain their own reserves. Over time, as local farmers became self-reliant, they stopped visiting the bank. But cultivators from across Maharashtra continued to seek her out, leading Popere to shift from a barter-based system to selling her seeds. At present, the seed bank has 116 varieties of 54 crops. She has committed to memory the details of each seed, its medicinal properties, uses, and standout characteristics. In a sense, she has become a living encyclopedia of traditional cultivars. Today, her seeds, especially vegetables, are in demand across India, in cities such as Delhi, Jaipur, and Bhopal, and have even earned an audience overseas.
In recent years, she has come to recognise that there is a limit to what can be achieved alone.
She traveled widely—on field visits, farm tours, fairs, and meetings—spreading awareness about the value of indigenous crop varieties. To dispel farmers’ doubts about the yield and profitability of indigenous crops, she experimented on her own farm, cultivating four traditional rice varieties alongside four hybrids under identical conditions. The results were striking: traditional varieties produced complete grains, while the hybrids struggled without chemical fertilisers. This comparison helped farmers see the resilience of local crops, which also withstand droughts and pests better.
Through such demonstrations, she was also able to win over Kombhalne’s women. Around 2,000 of them became associated with the Kalsubai Parisar Biyane Sanvardhan Samajik Sanstha’s seed bank, of whom more than 400 are actively involved in seed production. She has also unified groups of tribal women to work together through the establishment of self-help groups.
In 2020, Popere was awarded the Padma Shri—a turning point in her farming career, amplifying her vision and labour beyond Maharashtra. Six years on, her seed bank is lined with awards and trophies, though she emphasises that they have done little to change her financial reality. “I worked hard before, and I continue to work hard now. If you work, the seeds will come—and only then can you share them with others,” she says.
For Popere, every step forward was accompanied by struggles. In the early years, she endured relentless criticism and violence from her own family. Training trips meant to upskill and empower her often deepened tensions at home; her husband’s anger could be flared by the smallest things. Many nights, exhaustion and despair nearly pushed her to give up. But her father’s words of encouragement echoed in her mind each morning, and she pressed on, drawing strength from them. She knew stopping was never an option for her community and the seeds.
She has committed to memory the details of each seed, its medicinal properties, uses, and standout characteristics.
In recent years, she has come to recognise that there is a limit to what can be achieved alone. Troublingly, there have been times when the native seeds handed over to farmers have not been conserved after harvests. But every disappointment is matched by pleasant surprises. She recalls a meeting with a Satara-based farmer who purchased a small packet of wheat from her bank which cost barely Rs. 30. Instead of rushing into sowing the seeds, he spent months investing in preparing his land the way Popere had recommended, slowly undoing the damage caused by chemicals and reviving the soil’s strength. The resulting harvest astonished him: it yielded 30 kg of grain.
Overjoyed, he told her that he shared 10 kg of seeds with fellow farmers so the variety could spread, and saved another 5 kg to sow again in his own field. “If more farmers did what he did,” Popere says with conviction, “toxin-free, indigenous produce would once again find its way to every plate.”

Art by Jishnu Bandyopadhyay
Also read: Babulal Dahiya makes every grain of rice count
{{quiz}}

While breastmilk is essential for newborn infants, toddlers benefit from a balanced diet
Parenting can be an endless creativity contest—beetroots disguised into cutlets, spinach blended into a parantha, and vegetables shrouded in sauce and wrapped into a roll. All to serve one purpose: providing a child with adequate nutrition in a world where intense marketing and easy access to ultra-processed foods do their best to lead them astray.
The world, and India, is seeing an unprecedented rise in the number of obese children. In the span of 15 years, India has witnessed a 127% increase in the number of obese children under 5 years, and a 125% and 288% increase in the number of obese adolescent girls and boys respectively.
Given this current scenario, while kids may be tempted by fast food, conscientious parents may get influenced by the health food marketer’s current favourite: protein. How much protein do our kids really need as they grow up?
A child’s nutritional journey begins before their first breath. Pregnant women are advised to have an additional 350 calories daily during their second and third trimesters. These additional calories should include a supplementary 8g of protein during the second trimester and 18g of protein during the third trimester. This additional protein is crucial for foetal growth, reducing the risk of stillbirth and low birth weight. Following these recommendations usually results in 14-15% of the total energy being derived from proteins. Any protein supplementation should not cross more than 20% of the total calories consumed, to prevent adverse effects like restricted foetal growth.
Any protein supplementation should not cross more than 20% of the total calories consumed, to prevent adverse effects like restricted foetal growth.
These needs augment to an additional 600 calories of energy and 13.6g of proteins during the first six months postpartum. In the next six months, the requirements drop to an additional 520 calories of energy and 10.6g of protein. This additional consumption helps not only with optimum breast milk production, but also with maintaining the mother’s health.
For the first six months, exclusive breastfeeding can meet all the nutritional needs of the infant. In fact, not even water should be given during this time. In the next 6 months, the baby’s caloric needs are 650-720 kcal/ day (with 9-10.5g of protein), while breastmilk provides only 500 kcal/day (with 5g of protein). To bridge the gap, complementary foods should be incorporated two to three times a day alongside breastfeeding. In the initial 3 months, it should be food with a paste-like consistency, such as a fruit purée. Nine to 12- month-old babies can slowly progress to grated vegetables, and by the time they turn 1, they can start having more solid foods like khichdi and boiled eggs. While it is advised that mothers continue to breastfeed for the first two years, formula milk is a reality in India for a myriad reasons.
But in today’s context of mixed feeding, a baby consuming formula beyond their first month is likely having more protein than they need.
For any parent supplementing their child’s diet with formula, it is important to understand the chosen formula’s protein content. Unlike human milk, the protein concentration of formula is constant, and is designed to meet the needs of infants at all times, including the first month when protein needs are highest. The formulation errs on the side of excess because formula milk was created as a life-saving substitute for breast milk, specifically for mothers who couldn’t breastfeed. But in today’s context of mixed feeding, a baby consuming formula beyond their first month is likely having more protein than they need.
Also read: India’s first meal crisis: Is the rise of formula threatening breastfeeding?
In their toddler years, children continue to require more protein than adults in absolute terms: a 5 year old is advised to have 2.5 g per kg of bodyweight daily compared to the approximate 1 g per kg of bodyweight for the average adult. However, a balanced diet is enough to meet these needs for one simple reason: children weigh much less, and thus the actual net protein intake recommended for them might not even equal the recommended intake for an adult. Small inclusions in the diet can easily cover these needs: a cup of milk or half a cup of lentils contain 8g of protein, an egg or one slice of cheese has around 6g of protein, and 100g of greek yoghurt or 40g of chicken contains 10g of protein.
| Children’s age | Recommended quantity of protein per day [in grams] | Recommended quantity of protein per kg of bodyweight [in grams] | Percentage of total energy |
|---|---|---|---|
| 1-3 | 38 | 2.9 | 13.69% |
| 4-6 | 46 | 2.5 | 13.43% |
| 7-9 | 59 | 2.3 | 13.80% |
| 10-12 Boys | 76 | 2.1 | 13.63% |
| 10-12 Girls | 70 | 1.9 | 13.59% |
| 13-15 Boys | 95 | 1.8 | 13.28% |
| 13-15 Girls | 81 | 1.6 | 13.44% |
| 16-18 Boys | 107 | 1.6 | 12.96% |
| 16-18 Girls | 85 | 1.5 | 13.65% |
Also read: The science behind high-protein milk: How it differs from whey powder
Children have the same protein requirements regardless of gender until puberty. Thereafter, boys are recommended more grams of protein for the same body weight, owing to a higher metabolic rate and a higher muscle mass. Even at a lower absolute intake, girls have been recommended to incorporate protein as a larger percentage of their total caloric intake as compared to other macronutrients, especially once they turn 13.

Historically, a lot of recommendations on child nutrition have been based on detailed studies of breastfed infants and grown adults, with interpolations made for the intervening ages. A part of this research deficit is explained by the ethical considerations of conducting research on children. Regardless, this leaves a lot of questions unanswered about a child’s protein needs, including how activity levels and gender influence the recommended intake.
The beneficial impact of helping a child build a good relationship with their food will last much longer than any single meal plan can.
Throughout their childhood, but especially through these early years, protein is undoubtedly important for children. It is not without reason that the macronutrient is crowned as the building block of life: it plays crucial roles in growth, brain development, neurotransmitter production, nutrient transport, and immune response. That said, it would not help to err on the side of excess either: some papers have linked excess protein intake in children with an increased risk of obesity later in life.
Also read: Mess on my plate: India’s students are fixing their college diets
While research will emerge as the years pass and give us a more granular understanding of a child’s nutritional needs, the wisdom surrounding it stays constant. Childhood is when eating habits form. Rather than obsessing over any single nutrient, what will help is to build healthy eating habits, and develop the child’s taste for wholesome food. The beneficial impact of helping a child build a good relationship with their food will last much longer than any single meal plan can.
Artwork by Alia Sinha
{{quiz}}

A Geographical Indication tag and a grassroots revival led by dedicated farmers has ensured this beloved paddy variety remains alive
“For the past seven years—ever since I first tasted it—I’ve been buying Katarni rice from Subodh Choudhary, a farmer who grows it in a village in Bhagalpur,” says A. K. Singh, a Mumbai-based lawyer. “It’s far more aromatic and tender than Basmati. We bring it out only for special occasions—pulao, kheer, and festive meals.”
He swears by this indigenous grain. “Honestly, Katarni deserves to be prasad in temples.”
Singh is not alone. Across India, food lovers and chefs are rediscovering Katarni, a heritage rice from Bihar’s Bhagalpur region that once teetered on the verge of vanishing. Katarni is a landrace rice, signifying that it is a traditional variety that adapted and evolved naturally with its climate, with a great genetic diversity. This also means that landraces like it are more resistant to climatic shifts, droughts, and pest attacks.
Native to the fertile Chanan river basin, Katarni stands out with its small, slender grains, subtle aroma, and melt-in-the-mouth texture. With an amylose content of 21–24%, it offers a medium-to-high starch profile that contributes to its soft texture and appealing cooking quality. On cooking, Katarni rice blooms into fluffy grains that remain soft for hours. When compared to other aromatic rice varieties, it remains distinct owing to its biochemical traits shaped by Bihar’s unique climate and soil.
Native to the fertile Chanan river basin, Katarni stands out with its small, slender grains, subtle aroma, and melt-in-the-mouth texture.
Its fragrance is notably strong, scoring high on sensory tests; Katarni is genetically linked to the inactivation of the BADH2 gene—a trait shared with other aromatic rices like Basmati. It develops its characteristic aroma only when cultivated in a handful of river-fed blocks of Bhagalpur, Munger, and Banka, where farmers have carefully preserved it, harvest after harvest.
The cultivation of Katarni in this region began over a century ago. Its very name comes from ‘katarni’, the hooked awl used for stitching; the husk’s tip mirrors the sewing tool. Local experts say it is currently cultivated as a rabi crop in Bihar.
It is especially well-suited to prepare chura or beaten rice, which is consumed with curd ceremonially, often during the festival of Makar Sankranti. For generations, local households have reserved this rice for rituals and family celebrations, valuing it not only as food but as a cultural offering.

Also read: Why bajra, the ‘pearl’ of India’s millets, remains underutilised
But this fragrant grain almost slipped into obscurity. From the 1990s onward, its area under cultivation began shrinking rapidly. In the years leading up to the Economic Reforms of 1991, public investment in agriculture—especially irrigation and rural infrastructure—had declined. Reforms in the banking system affected agricultural credit granted to small and marginal farmers. These policies sought to introduce more competition into the agricultural sector, and favoured, by default, high-yielding breeds and bigger outputs. At this time, over thousands of landraces such as Katarni started vanishing. This drastic dwindling in their cultivation was dangerous, because it pulled down genetic diversity, exposing the rice crop to further threats.
High irrigation costs, competition from high-yield hybrid paddies, and the flood of adulterated imitations pushed many farmers away.
“There was a time when traders mixed Katarni with other rice varieties and passed it off at premium rates. Farmers got nothing from it,” recalls Subodh Choudhary, who has been cultivating Katarni as well as other other varieties like Tulsimanjari and black rice since 2016. Today, he runs direct-to-consumer sales (buyers like Singh approach him directly) to preserve Katarni’s identity.
While earning a GI tag afforded Katarni legal protection and even reduced imitation in the market, it has not guaranteed steady returns to farmers.
Even as the years left the Economic Reforms behind, the cultivation of Katarni suffered. In 1995, massive floods in Bihar’s Banka district caused field silting and sand excavation in the Chandan river. Adulteration eroded market demand locally and globally, pushing area under cultivation down significantly since 1991-92. Cultivation remained minimal and unstable.
Things changed in 2018, when Katarni was granted a Geographical Indication (GI) tag. The recognition tied its identity to the climate and soil of Bhagalpur, Banka, and Munger districts. This not only protected farmers, but also offered the rice a good chance at revival. The GI tag provided Katarni farmers an exclusive right to cultivate this rice, and continues to protect them. ‘Bhagalpuri Katarni Rice Utpadak Sangh', a registered farmers’ society, has been awarded the ability to utilise this GI tag by Intellectual Properties Rights, Govt. of India.
This recognition of authenticity has also helped producers enhance sales within and outside the state. “With the GI tag, people know what they’re buying. And that awareness is pushing demand,” says Choudhary.
While earning a GI tag afforded Katarni legal protection and even reduced imitation in the market, it has not guaranteed steady returns to farmers. One of the several challenges it faces has to do with the increasing preference for high-yielding varieties in the market; Katarni’s yields remain lower than other paddy crops, and younger farmers often abandon it for more profitable options. A recent structured survey of Katarni farmers in Bhagalpur revealed that they struggled with technological constraints, access to high-quality seeds, and the rising price of fertilisers.
Yet, its demand is slowly building again—driven by heritage-conscious consumers, artisanal food movements, and farmers like Choudhary who want to keep it alive. As frequent buyer Singh insists, “Katarni isn’t just rice—it’s part of our cultural memory. That’s worth protecting.”
Also read: Protecting place and power, not people: The trouble with GI tags
“Currently, Katarni is grown on 2,000 acres in Bhagalpur and 1,000 acres in Banka. Its cultivation cost is approximately 25% lower than that of hybrid rice varieties, and farmers earn well from selling puwal (paddy biomass) too, adding another layer of economic value to this heritage grain,” informs Prabhat Kumar, Deputy Project Director at AATMA (Agricultural Technology Management Agency), Bhagalpur.
Choudhary, a farmer-turned-agri-entrepreneur, has spearheaded a grassroots revival. Alarmed by the variety’s decline due to market adulteration and reduced cultivation, he founded Agro Heritage Pvt. Ltd. in 2018, with support from Sabour Agri Incubators and Bihar Agricultural University. His mission: restore purity, dignity, and market value to Katarni. He distributed authentic seeds, expanded cultivation, and built fair-market channels that rewarded quality. His efforts addressed systemic challenges like poor infrastructure, lack of access, and institutional neglect, while promoting organic farming and agri-incubation.
Its cultivation cost is approximately 25% lower than that of hybrid rice varieties, and farmers earn well from selling puwal (paddy biomass) too, adding another layer of economic value to this heritage grain
Manish Kumar Singh, who comes from a lineage of farmers from Abha Sultanpur in Munger district, began his professional journey as a teacher. But when the COVID-19 pandemic forced the closure of his educational institute in 2021, he pivoted decisively to agriculture. He started by cultivating Katarni on 20 bighas, and soon leased an additional 30 bighas to scale up. Today, he heads the ‘Jardalu and Katarni Agro Producer Company’, a farmer-led initiative focused on boosting smallholder incomes through authentic Katarni cultivation and improved market access.
In India’s rice heartlands, farmers face a cultural and economic crossroads: choosing between Katarni and Basmati. Katarni yields just 1.8–1.9 tons per hectare and is prone to lodging—the bending over or falling of the rice crop before harvest; it suffers from a fragile stem, making it highly susceptible to lodging under wind, rain, or excessive nitrogen. (New semi-dwarf lines may reach up to 3 tons.) Basmati varieties like Pusa 1121 and 1509 offer 4.5–6 tons per hectare, making them economically attractive.
This vulnerability often results in low yields far below those of modern dwarf hybrids. To address these limitations, the Bihar Agricultural University in Sabour initiated a breeding program to develop an improved landrace with reduced plant height, medium maturity, and higher productivity. The outcome: Sabour Katarni Dhan-1 (BRR 0215).
The rising demand for Katarni, now priced at Rs. 135 per kilogram, has triggered the influx of a counterfeit variety: Sonam.
“We began work on the variety in 2013,” says Dr. Mankesh Kumar, Associate Professor-cum-Senior Scientist (Rice Breeding), Department of Plant Breeding and Genetics. The variety was referred to the State Varietal Release Committee (SVRC) for a national-level recommendation earlier this year.
“Using molecular marker-assisted backcross breeding, we developed several semi-dwarfs, medium-duration lines that retain the exquisite grain quality of Bhagalpur Katarni. These lines closely resemble the original Katarni type. Multilocation trials across India, including GI-designated areas, have shown encouraging results, with yields reaching 46–48 quintals per hectare in farmers’ fields.”

The rising demand for Katarni, now priced at Rs. 135 per kilogram, has triggered the influx of a counterfeit variety: Sonam. This is a visually similar but lower-grade substitute that sells for just Rs. 35 per kilogram. “Millers are adding a flavouring agent—propylene glycol—to Sonam rice to mimic the taste of authentic Katarni,” explains Chaudhary. “This synthetic, colourless, and nearly odourless liquid is widely used to enhance flavour.
Katarni’s revival highlights the power of community action, GI tag-led protection, and sustainable farming in preserving heritage crops. As dedicated farmers and rising consumer interest drive demand, it stands as a symbol of cultural pride and economic opportunity—and its ongoing protection ensures that this unique rice will endure for future generations.
Also read: Babulal Dahiya makes every grain of rice count
{{quiz}}
Please try another keyword to match the results